Abstract
Introduction: While many older patients (pts) with AML attain complete remission (CR) after treatment (Tx) with intensive chemotherapy (IC), most will relapse and overall survival (OS) is poor, especially for pts who are not candidates for hematopoietic stem cell transplant (HSCT). In the randomized phase 3 QUAZAR AML-001 trial (NCT01757535), oral azacitidine (Oral-AZA), a hypomethylating agent (HMA), significantly prolonged OS (24.7 months [mo] vs 14.8 mo with placebo [PBO], P < 0.001) and relapse-free survival (10.2 vs 4.8 mo, respectively, P < 0.001) vs PBO in pts with AML in first remission after IC who were not candidates for HSCT (Wei AH, et al. N Engl J Med 2020;383:2526-2537). After discontinuing study drug, pts in QUAZAR AML-001 continued to be monitored for OS even if they received subsequent AML Tx. Given the high rate of relapse in older pts and the limited Tx options for relapsed/refractory AML, it is important to evaluate the impact of Oral-AZA maintenance Tx on the clinical outcomes for pts who received subsequent AML Tx after relapse.
Methods: Eligible pts were aged ≥ 55 years, had AML with intermediate- or poor-risk cytogenetics at diagnosis, Eastern Cooperative Oncology Group performance status score ≤ 3, and had achieved a first CR or CR with incomplete blood count recovery (CRi) after IC ± consolidation Tx. Within 4 mo of achieving CR/CRi, pts were randomized 1:1 to receive Oral-AZA 300 mg or PBO once daily for 14 days per 28-day cycle. Randomized Tx could continue until pts developed relapse with > 15% blasts, unacceptable toxicity, or HSCT. These post hoc analyses assessed Kaplan-Meier (KM)-estimated OS and 1- and 2-year survival rates for pts who received subsequent AML Tx after relapse, both from the time of study randomization and from the time of receipt of first subsequent Tx, until death by any cause. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models stratified by age, cytogenetic risk, and receipt of consolidation Tx. These analyses were not prospectively powered to detect statistically significant differences between Tx arms.
Results: At data cutoff (September 2020), 137/238 pts (58%) in the Oral-AZA arm and 172/234 pts (74%) in the PBO arm received subsequent AML Tx after discontinuing study drug. Demographic and disease characteristics at baseline in this subgroup were similar between the Oral-AZA and PBO arms and comparable to those for all pts in the trial (Wei AH, et al. N Engl J Med 2020;383:2526-2537). Pts from both Tx arms received similar classes of first-line subsequent Tx; 43% of pts in the Oral-AZA arm vs 45% in the PBO arm received IC, 50% vs 46% received lower-intensity therapies (including 25% vs 31% injectable HMAs), and in both arms 6% received supportive care and 1% received other Tx. In the Oral-AZA arm, 13 pts (9%) went on to receive HSCT, compared with 31 (18%) in the PBO arm.
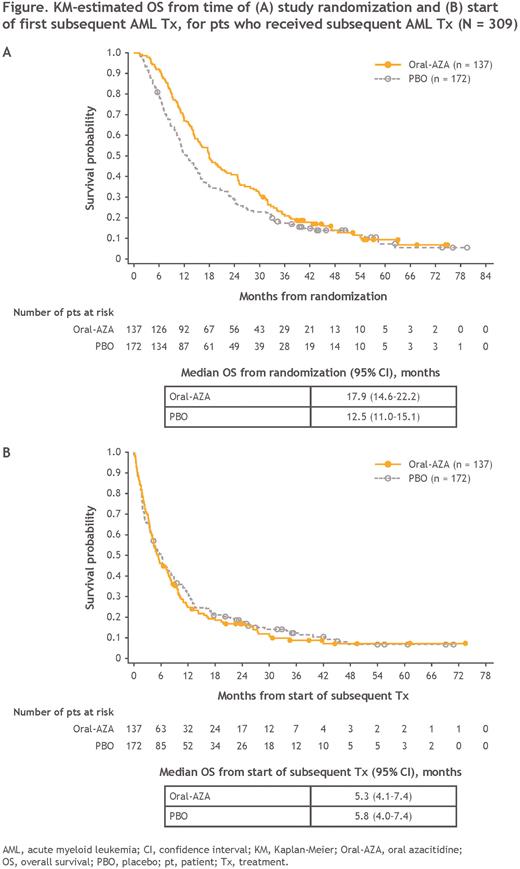
At a median follow-up of 51.7 mo, OS from the time of randomization for pts who received subsequent Tx was nominally prolonged with Oral-AZA vs PBO (median, 17.9 vs 12.5 mo, respectively; HR, 0.79 [95% CI, 0.62-1.0]) (Figure A). KM-estimated 1-year survival rates were 67% for Oral-AZA, compared with 51% for PBO, and 2-year rates were 41% and 29%, respectively.
Median OS from the time of first subsequent Tx was similar in the Oral-AZA and PBO arms: 5.3 and 5.8 mo, respectively (HR, 1.1 [95% CI, 0.85-1.4]) (Figure B). Estimated 1-year survival rates from first subsequent Tx were 25% in the Oral-AZA arm and 31% in the PBO arm, and at 2 years, survival rates were 17% and 18%, respectively.
Conclusions: Pts who received Oral-AZA during first remission, followed by subsequent Tx, showed a trend for improved OS from the time of study randomization compared with pts who initially received PBO. Survival outcomes from the start of first subsequent Tx were not meaningfully different between Oral-AZA and PBO. This suggests that Oral-AZA maintenance Tx can extend the duration of remission without influencing the clinical benefit of subsequent AML Tx.
Disclosures
Ravandi:Xencor: Research Funding; AstraZeneca: Consultancy; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Amgen: Honoraria, Research Funding. Montesinos:Novartis: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Astellas: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Menarini/Stemline: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Kura Oncology: Consultancy; Otsuka: Consultancy; Incyte: Consultancy; Ryvu: Consultancy; Nerviano: Consultancy; Beigene: Consultancy. Pfeilstöcker:Bristol Myers Squibb: Research Funding, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Sobi: Consultancy; Takeda: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Papayannidis:Abbvie: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Blueprint: Honoraria; Incyte: Honoraria; GlaxoSmithKline: Honoraria; Bristol Myers Squibb: Honoraria. Dombret:Servier: Honoraria; Incyte: Honoraria. Lai:Bristol Myers Squibb: Current Employment; Daiichi Sankyo: Ended employment in the past 24 months. Petrlik:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Prebet:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Döhner:Pfizer Inc.: Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding; Novartis AG: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Berlin-Chemie AG: Consultancy, Honoraria; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Kronos Bio, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal